Space Travel: Humanize a Tardigrade
The humble Tardigrade makes the news so often for its amazing survival on the moon, or in space, or exposure to 5,000 Gy of gamma rays. Now scientists propose to give humans the tardigrade’s radiation-resistance genes to help us survive in space. Specifically, the Dsup gene, which was shown to protect tardigrade DNA. What do you think? Would you like to travel space with a gene from a microscopic creature that looks “like a cross between a caterpillar and a naked mole rat”? (This breathless prose from the usually staid journal Nature.)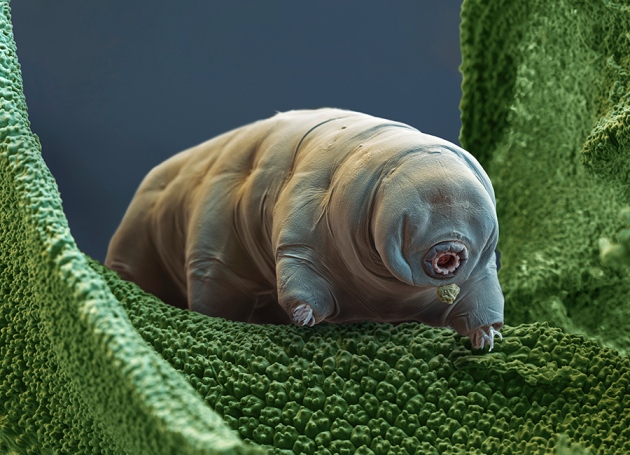 Eye of Science/Science Photo Library
Eye of Science/Science Photo Library
This story reminds me of a biochemistry conference in Virginia that I attended twenty years ago with my MD/PhD student Lisa Maurer, where Frances Collins held forth in glowing terms about the Human Genome Project. At the end of the lecture, I asked a question: Would Dr. Collins favor humans acquiring cancer-resistance genes from the genome of our close relative the chimpanzee? (Chimps have stronger immune systems and resist cancer far better than we do.) Collins, however, stared in shock. His gaze swept the vast audience of chemists, as if to say, who let her in here?
Fast-forward: How far we have fallen, to contemplate acquiring radiation-resistance genes from the lowly tardigrade (sic–I just taught in intro bio that there is no such thing as a higher or lower organism). FYI, here are the data on the gene of interest, Dsup.
This figure from Takuma Hashimoto’s paper in 2016 shows that Dsup protein (green) coincides with DNA (violet) in the nucleus of human embryonic cells in tissue culture. The idea is that the Dsup protein somehow binds DNA and protects it from damage by ionizing (bond-breaking) radiation.
I’m not sure though how well this would work. I suspect that the tardigrade’s superior radiation resistance (along with dessication and starvation survival) derive from more than one “superior” gene.
Suppose instead we try the reverse: Put human genes into the tardigrade. Then suit up the humanized tardigrades as our space travelers.
This is not so far-fetched as it sounds. Consider that we now “humanize mice” all the time, by providing for instance an entire human-cell immune system or a human-derived microbiome (microbial community). We market them like commodities. “Humanized mice define Knockin models in which a mouse gene is replaced by a human gene…. Phenocopy human diseases for drug development and safety studies.”
Sure, tardigrades are small. Mice are pretty small too, small enough to feed your pet tarantula. Yet still, mice possess a remarkable portion of our intelligence, feelings and responses. Human-like cognition might yet be scaled down to microbots or even microbes, as in Brain Plague. And they might be the only sentient beings to survive the coming climate apocalypse, let alone space travel.
Bioprinted Heart
3D printing of living organs takes a step forward, with a new printing process reported in Science. The organs are printed out as a scaffold of collagen (cell matrix connective protein). The collagen is printed as a “bioink” of hydrogel (protein gel in liquid form) containing suspended living cells. The hydrogel needs a signal, usually light rays, to solidify. After the organ is incubated, over time the cells grow, replacing the collagen.

Bagrat Grigoryan and colleagues used this method to form an artificial lung, complete with air sacs and ducts, and blood vessels. To solidify the organ material, they used an interesting approach of incorporating food dye molecules. The dye molecules absorb light at specific wavelengths to signal solidification. The dyes used include naturally occurring substances such as the turmeric pigment curcumin, or the blueberry pigment anthocyanin.
Adam Lee and Andrew Feinberg, and colleagues at Carnegie Mellon, printed out a 3D heart, the size of a newborn’s. They used a different set of methods (and patents of course) to print a matrix of collagen microparticles whose fusion is triggered by a pH change. They achieved remarkable size resolution, with voxels (3D pixels) of detail down to 20 micrometers (millionths of a meter). What is amazing is the complexity of detail of convoluted tubules that can be “printed” with these methods.
And yes, a heart ventrical does start to beat.
Just a Few Human Cells
Mice move over: Human-chimp chimeras are exactly what the Elysians make in Daughter of Elysium.
Of course, this story has all the elements of the “forbidden science” theme: It’s not done here (of course not) but in China (how many reports of humans cloned in China, back in the day). If human-chimp chimeras are actually made, it shouldn’t be hard; actually much easier than human cloning. Human-mouse chimeras have been made already, though not past the stage of early embryos.
Other animal chimeras have been grown to maturity, for example a goat with a sheep makes a sheep-goat chimera. This is different from a hybrid, that is a sheep inseminated by a goat or vice versa, where every cell in the body has half sheep chromosomes and have goat. In a chimera, each cell comes from one parent or the other, but the cells are gemished throughout the organism in various ways. Chimeras are interesting because their sex cells come from one “parent” or the other. So a human-chimp chimera could theoretically produce human sperm or egg. Of course scientists say they won’t make chimeras that can do that.
Why make these chimeras? The same reason science fiction always offers for human clones: A source of transplantable organs.
So how much human does the chimera have to be before it expects human rights? What percentage? What organs? A human spinal column? Oops.
According to the news report, if mice-human chimeras were to be born, “it is very unlikely the animals, if brought to term, would take on human-like behaviour, but the animals might not behave like “normal” rodents.”
Human Brain in a Mouse?
Would it be ethical to construct a mouse with a human brain? It sounds too impossible to worry about.
Yet various kinds of “humanized mice” are not just possible, but routinely for sale at breeding centers such as Jackson Laboratories. For example, a mouse’s population of bone marrow stem cells can be replaced with human stem cells, generating a mouse with a “human immune system.” I put this in quotes because how human can the system be, with all its connections to other parts of the mouse body?
Nonetheless, these “humanized” models lead to medical advances in cancer and other medical research. And bacterial humanized mice—mice harboring human gut microbiomes—are the kind of research even Kenyon undergradates may contemplate.
The trouble is, humanity is seductive—addictive, if you will. No matter how much you humanize, a bit more human is always better. That was the background of A Daughter of Elysium; human hybrids led to slaves, and experimental developmental models, all to help humans live past a thousand years. But the more human, the better model… so suppose the model gets to be 50% human? 90%? 99%?
Would a mouse with a human brain be a human in a mouse body?
In 2000, a researcher first proposed to develop a mouse with human brain cells, using a mouse strain whose own brain cells are genetically programmed to die early in development. Bioethicists formed a committee to address whether this research was ethical. How seriously, I’m not sure. They deliberated for five years before concluding that “the question does need further work.”
Today, researchers study mice with human brain cells.
Jonathan Hasselmann and a large team at UC-Irvine, UC-San Diego, and McGill University, developed mice with human microglia—not neurons, but the neurons’ intimate defense partners. To do this, the researchers converted adult human tissues into pluripotent stem cells (the kind that could almost make a baby). The stem cells, capable of many developmental paths, also were engineered with a fluorescent protein (GFP) to tag them. Researchers injected these tagged, development-capable stem cells into the fetal mouse forebrain. Eventually, a region of the brain showed 80% human microglia.
The human-origin cells could be detected as dots of green fluorescence. Ironically, the green-fluorescent GFP itself originates from jellyfish, a third participant in this Franken-human construct.
The aim of this research? Finding therapies for Alzheimer’s disease, Parkinson’s, and other dread human conditions. There is no denying the need to treat and prevent these devastating conditions. And we inexorably approach every nearer the daughters of Elysium.
CRISPR Plus Herpes Gene Fixes Muscle
Those virus genes are all up to tricks again—treating rare diseases.
Muscular Dystrophy type 1A is a disease of wasting muscle, in early childhood. Skeletal muscle and nerves deteriorate for lack of a cell matrix protein LAMA2—that is, a kind of structural strap that holds cells together in tissue. To edit this protein is a challenge because the defect is rare; and because every infant born with it has a different rare mutation. One approach to thereapy is to edit the gene, using the CRISPR gene editor system.
CRISPR was derived from a system of bacterial self defense against bacterial viruses, discovered by Jennifer Doudna and Emmanuelle Charpentier, likely to win the Nobel some day.
But a treatment devised to edit one mutation won’t work for others, over 350 variants found so far. Also, CRISPR gene editing is risky, because errors in the editing process itself—called “off target effects”—can cause cancer.
So another scientist Dwi Kemaladwi devised a new approach.
Kemaladwi used a form of CRISPR-Cas9 editing enzyme that has its own mutation, preventing Cas9 protein from cutting the DNA. Instead, Cas9 binds a DNA sequence marked by a guide RNA (sgRNA) to locate a promoter DNA sequence. The promoter sits upstream of gene LAMA1—encoding a protein very similar to LAMA2. This is common in the human genome, that we find several slightly different versions of a gene. Kemaladwi thought that overexpressing LAMA1 might help compensate for the failur of LAMA2.
So Cas9-sgRNA can help locate a promoter for LAMA1. How do we then activate LAMA1 expression (making RNA copies for protein production)?
Activation is the job of a Herpes virus protein: VP64 (actually, four copies of herpes protein VP16).
So here is the Franken-gene construct, which yet another virus (adenovirus vector) has to deliver to the muscle cells:

ITR = inverted terminal repeats. Placing these at either end of the DNA map enable the whole piece to be inserted in the adenoviral vector (small viral carrier genome).
CMV = cytomegalovirus promoter, to express the Cas9. Cytomegalovirus is a herpes-related virus that causes most of our congenital birth defects; ironically, here it’s going to help treat a genetic defect.
3xFLAG = an antigen tag to help scientists visualize cells making copies of the viral vector DNA
VP64 = 4 copies of the gene encoding herpes activator protein
SadCas9 = Cas9 protein sequence from Staphylococcus aureus, better known as Staph infection or sometimes MRSA (methicillin-resistant Staph; flesh-eating Staph)
U6 = a promoter sequence especially effective for expressing small RNA (sRNA) such as the (guide RNA sgRNA) for LAMA1
What happened? In a mouse model, the viral vector was injected, where it infected muscle cells and expressed the LAMA1 protein. This image compares cross sections of the mouse scitiatic nerves, with or without the vector treatment. The treated muscle (lower image) shows thicker sheathes around the nerves, more normal.

The treatment looks promising in mice, so we’ll see if it can be developed for humans.
Deer Grow Antlers and Fight Cancer
Did you ever wonder how Rudolph grows new antlers so fast—and regenerates them? To say nothing of pronghorns, muntjacs, gazelles and buffaloes? The only completely regenerable organ found in mammals. And they don’t get cancer.
So what can we learn here about bone regeneration and fighting cancer?
A large group of scientists, mostly in China, are trying to find out. They are mining the Ruminant Genome Project.
First they did what’s become the obvious for everything:
• Map all the genomes. Sequence every cervid’s DNA and make a giant family tree.
• Find candidate genes. That is, which genes that act on development show consistent dependence on regenerable headgear? And maybe don’t appear in non-antlered cousins.
• Map transcriptomes. That is, the pattern of RNA transcripts actually copied from the DNA genomes during antler regeneration. Our technology now converts the RNA copies back into DNA, which gets sequenced on Illumina super-sequencing machines. The transcriptome tells us which genes of an organisms’ genome are actually getting used in certain tissues.
The phylogeny above shows how the relationships came out. Notice that two groups were included that had lost antlers through reductive evolution, the Moschidae and the Chinese water deer. This makes sense as a control—genes specific to antler growth should be absent or down-regulated in the bare-headed species.
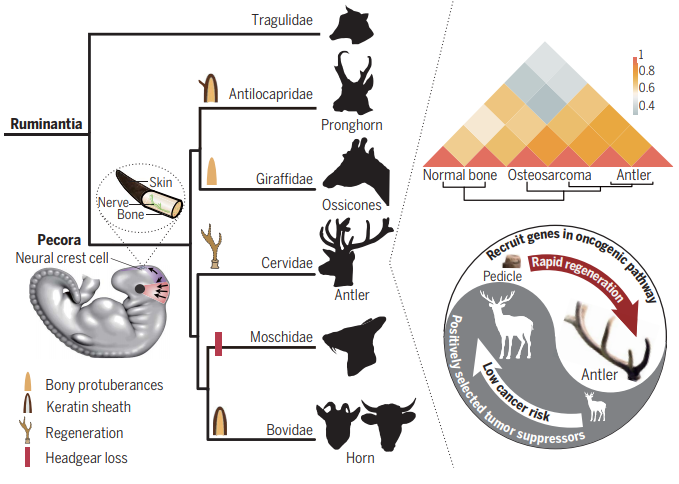
So what molecular results came out? Some fascinating clues.
• Horns or antlers expressed regulators of development in the neural crest, the earliest embryonic region of cells giving rise to bones and muscle as well as nerves.
• Amazingly, the genes expressed during antler growth have more in common with osteosarcoma (bone cancer) than with normal mammalian bone. In other words, deer use the same molecular tools of cancer to rapidly grow their antlers. Yet deer have less cancer than other animals. How do they regulate antler bone growth so tightly?
• Antlers are full of nerve fibers. The “nerve growth factor receptor” is highly expressed in growing antlers.
So have we cured cancer yet? A bit early for that, although the scientists assure us that “a lot of genes that exist in cows and goats and deer and sheep also exist in humans.” In other words, just maybe if you can’t yet regrow your hair, you might grow a prong or two. 😉
Two Scientists
This summer has been tough for me, with the passing of two scientists I knew well. The first is my father, the physicist John Slonczewski, 1929-2019. As Wikipedia helpfully says, “Not to be confused with Joan Slonczewski.”

Back in the 70s, John wrote the Britannica article on magnetism. A lifetime theoretical physicist at IBM, John invented equations for magnetic effects that are now used in all of our thumb drives and mobile devices. A video at the Computer History Museum describes his work.
John received the International IEEE Magnetics Society award for prediction of the spin transfer torque effect in magnetic thin films. He also received the 2013 Oliver E. Buckley Condensed Matter Physics Prize for a lifetime of work including MRAM, Magnetoresistive Random Access Memory. This kind of memory is the only kind that does not “wear out” and is non-volatile (does not go away when power is off).
Nominated for a Nobel, John was better known to us over the years for his fun-loving adventures with family, especially beloved Esther.

***********
My second heartbreak this summer was the loss of our Bacteria Lab graduate Sean Bush, 1994-2019, third-year medical student at Wright State who passed in a bicycle accident.

At Kenyon, Sean was a preternatural computer whiz who installed our lab’s supercomputer node that formed the foundation of our our current Bacteria Lab, our past six publications. He was also the most compassionate human on Earth, readily sharing his gifts with students ranging from Bacteria Lab to Wiggin Street Elementary.

He was still running our computer from med school, where he was on track to become a neurosurgeon. In life though you never know what’s round the corner, and Sean lived every moment as if it were the most important.
Spider-Toxin Franken-Fungus Fights Malaria

Where to begin, with this “Frankenstein of the week” story? The recluse spider, whose brew of toxins perhaps earns it an overly bad rep? True, humans occasionally pick up the Hybrid toxin, its name perhaps inspired by a Marvel superhero. Malaria is arguably the world’s most debilitating microbial disease, for human and animal death and morbidity, as well as economic impact. Every few decades another antimalarial med or insecticide is spread across the malaria belts of Africa and Asia, only to fail a decade later as resistant pathogens arise. Spread by the Anopheles mosquito, the parasite invades red blood cells, crowding the blood with Plasmodium parasites, and spreading to the bite of new mosquitoes.
Malaria is arguably the world’s most debilitating microbial disease, for human and animal death and morbidity, as well as economic impact. Every few decades another antimalarial med or insecticide is spread across the malaria belts of Africa and Asia, only to fail a decade later as resistant pathogens arise. Spread by the Anopheles mosquito, the parasite invades red blood cells, crowding the blood with Plasmodium parasites, and spreading to the bite of new mosquitoes.
The latest entrant into our malaria arms race is the fungus, Metarhizium pingshaense. This previously obscure fungus is known for attacking mosquitoes. Here we see a hapless Anopheles mosquito dying a gruesome death, thanks to fungal attack. The fungus paints the mosquito green, from a plasmid (DNA circle) that genetically engineered the fungus to express green fluorescent protein (GFP). GFP originally comes from a jellyfish, carried by a bacterial plasmid—before the GFP gene got immortalized by Roger Tsien and others, engineered into a rainbow of variously-colored fluorescent proteins.
So can we enlist Metarhizium as a weapon against the mosquito that carries killer plasmodiums? As vicious as the fungus appears, apparently it’s not strong enough to dent the vast mosquito population. But researchers don’t give up easily. Brian Lovett, entomologist at University of Maryland, teamed up with Etienne Bilgo, Sante/Centre Muraz in Burkina Faso (and a host of others) to engineer a fungus that produces the spider toxin.

Here, Etienne Bilgo is part of a team that tested the ability of a genetically engineered fungus to kill mosquitoes that can spread malaria.
How did they make the fungus-plasmid-spider-Hybrid hybrid? That feat was reported by Paula Calabria and her team at the Butantan Institute, São Paulo, Brasil. (You can see how badly people all over the world want to fight malaria.) First, they undertook some molecular sleuthing to identify the spider gene that encodes two toxins from spider venom. The genes were spliced together to encode “Hybrid” toxin. Originally their idea was to generate an anti-venom agent to treat people poisoned by a spider bite. The hybrid toxin would be used to raise antibodies that could neutralize spider toxins.
But other researchers found another use for this toxin, which effectively destroys an insect when expressed within the insect’s hemolymph (blood-like fluid). The gene encoding the Hybrid toxin was introduced into the fungal cells by use of a plasmid vector, a circle of DNA into which the Hybrid gene was spliced using restriction enzyme-cut DNA.
How to get the fungus into the mosquitoes? The recombinant fungus was suspended in sesame oil and spread onto black sheets within experimental living quarters. The mosquitoes were infected and died, or left few offspring. Moreover, the infected insects released fungal “conidia,” the spores that grow new fungi. It remains to be seen if this new virulent Franken-fungus can persist in the environment and effectively control malaria mosquitoes. And–avoid killing other insects the ecosystem needs.
Carbon Comes Home
Any serious plan to solve the carbon problem, to stop global warming before the planet cooks, must include carbon capture. That’s because, even as we replace CO2-releasing energy technologies, it won’t be enough: The CO2 up their already will continue to bake the planet, with irreparable harm for ocean food chains. What will it take to stop?
Somehow we must take CO2 out of the air and put it somewhere. But who trusts pumping a gas down into the Earth? A better way is to build the carbon into carbonate (carbon-oxygen) materials that are inert, and even usable for building construction. This was a premise of The Highest Frontier, where carbon is reacted into “carb” that (ironically) builds the tall seawalls that protect coastal cities.

Materials such as carbon fiber, and more are being invented.
This MIT lab claims to suck carbon into construction while releasing oxygen, like plants do. But building anything requires input of energy from somewhere. Can building carbon fiber be carbon-neutral?

Carbon Engineering thinks they have a comprehensive plan for carbon removal, and they’re starting now, with a plant in Texas. Their plan impressed the New York Times.
So how does it work? Inject base (hydroxide) into air with fans, and precipitate the CO2 into carbonate. (Where does the hydroxide come from? Takes energy to make.)
Calcium carbonate (Where does the calcium come from?) is then packed into pellets, converted into calcium oxide, or pumped into the earth. All these processes can store CO2, but they all cost energy from somewhere.
Ultraphyte thinks it’s great to start somewhere—but do the math on your thermodynamics. The laws of energy and entropy are unforgiving.
Phage to the Rescue
A bacteriophage (virus that infects bacteria) may have cured a patient dying of multidrug-resistant bacteria.
The patient, Isabelle Carnell-Holdaway of Faversham, UK, had a lung transplant, as a result of cystic fibrosis, a condition in which the cilia lining the lungs are paralyzed and bacteria build up. The lung transplant required suppression of the immune system. So a multi-drug resistant bacterium grew and spread throughout her body. The bacterium, Mycobacterium abscessus, is related to those that cause tuberculosis and leprosy—a really nasty pathogen.
But Isabelle’s mother had heard of “phage therapy,” an experimental treatment in which bacteriophages attack a pathogen.
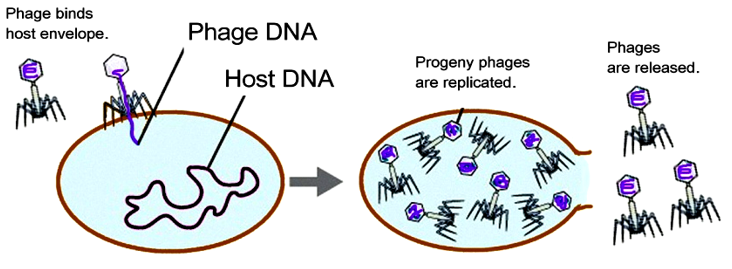
The phages have to be very specific to the bacterial species, which they inject with their DNA to program the cell to make more phage particles. The idea of phage therapy goes way back to the early twentieth century, as dramatized in Sinclair Lewis’s novel Arrowsmith. But no full-scale trials have ever been conducted. There were always antibiotics that worked more simply, and had a broader spectrum of activity.
The problem was, where to find a phage that would specifically infect M. abscessus? Doctors turned to Graham Hatfull, a professor at University of Pittsburgh who has worked with large teams of undergraduates to isolate new phages. This project, which now reaches students all over the world, is called the Science Education Alliance Phage Hunters Advancing Genomics and Evolutionary Science (SEA-PHAGES).

Hatfull searched his collection of ten thousand undergraduate-discovered phages. In the collection he discovered three phages that could attack the specific pathogen that had infected Isabelle. One of the three phages, called Phage Muddy, had been discovered from a rotting eggplant. That gives you some idea of what phage discovery is like.

Since no one know which phage would actually work (destroy the infectious bacteria despite Isabelle’s suppressed immune system) she was treated with a combination of all three phages. This was kind of a “Hail Mary” attempt, something you do only when all other treatments have failed.
Isabelle’s infection receded surprisingly fast, then cleared up almost entirely. While the story is not done yet, it looks like phages gave her a miraculous life extension. Time to revisit Arrowsmith.