Newborn Genetic Diagnosis—Guinness World Record
In 2003, the Human Genome Project claimed completion of the first DNA sequence of “the” human genome. Just fifteen years later, hospitals can sequence an entire baby’s genome literally overnight. Typically 19 hours, at the Rady Children’s Institute for Genomic Medicine, holder of the Guinness World Record, up there with the world’s largest origami rhinoceros and the most balloons popped by a dog.
Why is it important to sequence the DNA of a newborn?
4%, that is 1 in 25 newborn children has a genetic defect. Think about it—How many people you know have this in themselves or their families?
For treatment, the first days, even hours, are crucial. The sooner therapy can start, the greater the chance that an infant can have a normal life. This is true for certain conditions, such as phenylketonuria (PKU), cystic fibrosis, immune deficiencies, and tendencies for early cancer. PKI has always had a simple biochemical test, required by law at birth. But what of thousands of other conditions? Sadly, many of those still have no cure. But increasingly, knowing the defect early means help is on the way.
To sequence a genome and get useful information that fast requires chip technology and enormous computing power.
First, there is Illumina DNA sequencing, in which fragments of DNA are stuck to special “start” sequences at either end of the double strand. A DNA polymerase enzyme extends each strand, by fitting nucleotides to its complement. Eventually, all the short sequences are read and assembled into a whole genome.
Next, a computational pipeline that screens the three million base pairs and sifts out the likely errors that might have significance. Surprisingly, the fast majority of mutations have no effect at all on our health; even some “lethal” ones, whose compensatory mechanism may be unknown. Each of us has about 150 mutations not present in our parents. So, finding the lethal one may be quite a trick.
Studies show that genome sequencing can actually save money, as well as lives, by enabling treatment before a condition does irreparable damage.
So in the future, should we screen all newborns for entire genomes at birth, not just PKU?
That is another question, with many complex considerations.
CRISPR for Cancer?
CRISPR, the gene editing tool that sounds like something out of a fridge, is now talked about for every genetic disease and just entered a clinical trials to treat cancer.
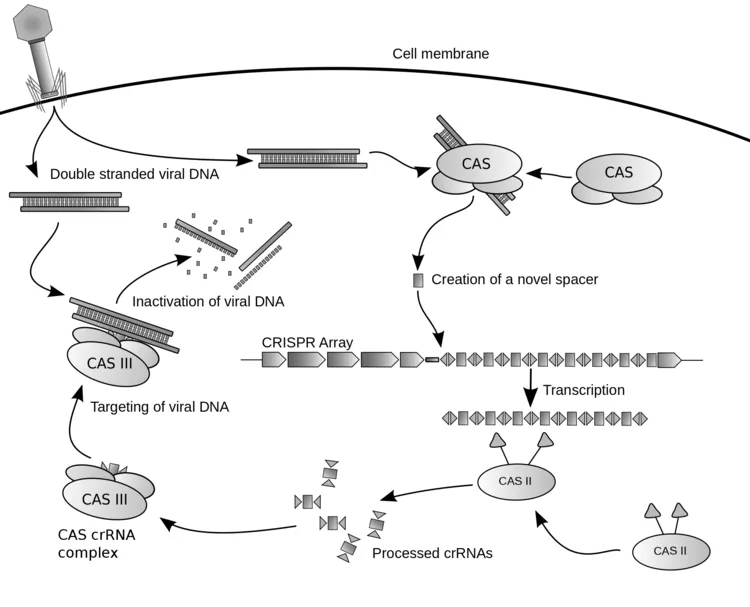
What is it? In a cell, a complex of proteins and RNA that can cut a gene, in some cases cutting out one gene and splicing in another. Big business, leading to mega patent disputes. Yet it all started from a relatively modest NSF grant to Jennifer Doudna about bacterial DNA.
As Ultraphyte presented before: CRISPR stands for
Clustered (short DNA sequences)
Regularly
Interspaced (with bits inbetween)
Short
Palindromic (read the same forwards and back)
Repeats.
So where did this DNA-editing machine come from? It evolved over millions of years in bacteria, as a bacterium’s immune system. The clusters of short repeated sequences appear in the bacterial DNA. Each short sequence has been copied from the viral genome of a previously infecting virus.
Some generations before, a bacteriophage (bacteria-infecting virus) injected its DNA into the cell. The DNA sequence was recognized as foreign by the CAS complex, which is a protein/RNA machine that makes RNA copies of the infecting DNA sequence. The CAS RNA copies then cause the cell to (1) degrade the viral genomic DNA; (2) make short copies of viral sequence and insert them into host. These short copies (the “palindromic repeats” of CRISPR) then serve to generate future CAS RNAs that recognize the virus when it infects again–a kind of bacterial immune system.
Now what molecular biologists are doing is to engineer the CAS complex (and related protein machines, from different bacteria). Incredibly, the CAS machine has been made to work inside a human cell, where it can snip and edit one gene. The CAS needs to be engineered for extreme accuracy, to cut only one place in a large human genome, and to avoid “off-target effects” (unknown other ways it could mess with a genome). For example, at Duke University researchers have found a way to modify the shape of the RNA part of the machine so as to make the cutting more specific to the DNA sequence. This is really important when you are trying to fix one gene out of 3 billion base pairs.
The first clinical trial for cancer has been approved, at the University of Pennsylvania, for treatment of multiple myeloma. Multiple myeloma occurs in white blood cells called B lymphocytes, or B cells. These B cells develop in the bone marrow, and certain clones proliferate when stimulated by an antigen to make antibodies. Then these antibody-producing cells develop into plasma cells, which are supposed to produce antibodies in the blood to combat infection. But when regulation fails, too many plasma cells are made, and they make abnormal antibodies that clog the blood.
Normally, the body needs to make T lymphocytes to regulate the B cells, but in multiple myeloma this regulation fails. So the aim of CRISPR therapy is to restore the patient’s own T cell regulation. This is done by:
–Remove some of patient’s own T cells for engineering (by autologous donation, giving cells back to oneself afterward)
–Giving the patient’s T cells a gene called TCR that recognizes a cancer surface protein called NY-ESO-1, found on myeloma cells. Now the T cells will be able to recognize and eliminate the cancer cells.
–To give the gene to the cells require a vector DNA called a lentivector. This lentivector was engineered from a lentivirus, originally the same HIV virus that causes AIDS. Ultraphyte has discussed lentivirus before; and The Highest Frontier shows lentiviruses as a future everyday therapy like aspiring. Amazingly, lentiviruses are now a standard approach for developing new cures.
–Use CRISPR to edit three other genes of the T cells, altering their function to enable these engineered T cells to attack the myeloma cells.
If that all sounds like a mouthful, it is. The result could be a miracle cure for an incurable illness. As you might imagine, though, as such cures become routine, increasing complexity means increasing development costs, and costs to your insurer. Something to think about as we develop these cures: Who will be able to afford them?
Galápagos–Volcanoes Young and Old
The Galápagos islands are an unfinished project of volcano building, perhaps going on for the past 90 million years. A plate of mantle moves southeast above a magma chamber that fountains new rock, creating new land. The result provides the equivalent of a testing ground for evolution of new species.
Here on Isabela Island, Punto Moreno, we clambered over a relatively young lava field, just 300 years since it solidified. Much of it remains smooth and black, barely a trace of soil. Only the marine iguanas, which long ago evolved black coloration to hide against the rock, here can be seen like drops of lava come alive to slither down and hunt for algae.
The lava still has the “ropy” texture of molten rock flowing down the mountain.


During the flow, the various metals within the rock separated out by density, especially iron oxide. The separations led to layers of color, particularly red iron, and yellow.


Different islands formed from different offshoots of the magma chamber–where the metals had already separated. The lava forming Rábida Island had 80% iron, which oxidized to red and crumbled into red grains of sand. Here too the animals must adjust.

Galápagos–What They Never Tell You
Back from the Galápagos Islands, I’m still getting the “sea legs” feeling out of my brain. I’ll take just a moment to reveal things they never tell you in the nature books.
The animals of course are all protected, off limits. Many signs tell humans to keep six feet away from them. What does that look like?

After three days of feeding us elegant ceviche (raw fish soup) we visited this outdoor market where the fishers were selling their catch of the day. With the help of a sea lion and three pelicans, one of them right on the counter with the cutting board. Most hygienic.
In actuality, there is a complex balance amongst the nature park, the long-time human inhabitants, and the tourists. Each constituency gets a portion of land and time available. 99% is off limits, protected park, but there is still plenty of access. And some of the animals don’t seem to mind.

Every sea lion feels entitled to the nearest level ledge where it can haul off and rest, sometimes a mother nursing a pup. At the beaches, every bench is full of a sea lion. As a result, I avoided sitting on any outdoor bench everywhere (knowing what kind of poop had probably landed there).
Speaking of poop, that is an unexpected hazard of bird watching on the boat. We lay back for hours watching the frigate birds circle overhead, where they enjoyed the air bumped up by the moving boat. The two upper birds are male; you can see the red throat pouch if you look closely. I heard a scream, “Poop on my I-pad!”

At night the boat attracted baby animals of all sorts–the light or the effluents, I’m not sure, but there they were, baby sharks, baby sea lions, and baby turtles. The baby sea turtles had probably hatched that very day, from the egg nests on the beach surrounded by protective signs.
To the Galápagos Islands
The next two weeks I’ll be away on a tour of the Galápagos islands. I’ve read and taught about the islands, including Vonnegut’s novel of that name, for the past twenty years. So it’s exciting to actually get there.
The islands have formed out of volcanic activity over the past eight million years; some are barely tens of thousands of years old. Volcanism continues today. This image comes from a remarkable video, Wild Galápagos.

What is remarkable is the many intricate relationships between creatures that evolved over a short period of time. Consider the fierce-looking lava iguana, an iguana that colonizes the harsh bare rocks of solidified lava.

What do these fierce creatures eat?

The iguana grazes beneath the seawater on green algae, nourished by nutrient-rich currents from Antarctica. But then the algae must survive the powerful surf that could dash it against the rock. Remarkably, the iguana climbs back up.

While they try to rest, the iguanas are bothered by flies. So they let smaller lava lizards climb up to hunt the flies.

What about love? Everyone’s heard of the blue-footed boobies who will mate with whoever’s got the bluest feet at the moment.
But waved albatrosses pair for life, though they migrate apart across the ocean. They return to these islands to reunite. Here, a pair of waved albatrosses rediscover each other after six months. My, are they excited!
They mate and raise a chick together–unless the wily mockingbirds get their egg first. A harsh existence, but fascinating.
4D Printing of Life?
We’ve all heard of 3D printing, the computer-controlled buildup of a material into a three-dimensional form. So what is 4D printing? Basically, the buildup of a material that has special functional properties.
A simple example would be a material that can be induced by a signal to deform or fold itself. The signal can be heat or water. Amazingly, the heat-deformable object shown retains its structural strength and load-bearing capability, even after it’s deformed.
Still other objects can be induced to fold into a precise 3D form. The MIT Self-assembly lab is famous for such devices.
What gets more interesting is when the printed substance consists of living cells and tissues. For instance, we can now print out replacement corneas for cornea transplant. Hearts and thyroids are on the way.
So at ICFA (the fantasy conference) we wondered: What would happens if you could print out an entire human being? “Clone” is a questionable term, because it has always referred to development of an organism from a single-cell zygote; in effect, a delayed twin. A 3-D printout however would have all the structure found in the copied adult—scars, memories encoded in neural connections etc. What rights would such a printout human possess?
Clone with Joan at ICFA 2019
We couldn’t clone Gay and Joe, but we sure tried! Jeanne and Johnna, too. What a great time we had–from human CRISPR to health-improving lentiviruses, microbial invention of metazoans to 3D-printed humans. Gut bacteria fight depression, if you’re Belgian or Dutch. And the truth about the hygiene hypothesis.



Meanwhile, who could beat the diverse fauna of our setting.



See you at ICFA 2020!
Brain Stroke Zaps Gut Bacteria
The gut-brain axis means your intestinal bacteria can influence the brain. But does the brain talk back and regulate the gut?
According to West Virginia University researchers, that is what happens.
In experimental animals, a brain stroke (brain cells die due to injury) leads to disorganization of the gut lining, for weeks afterward. And the proportion of “good” bacteria decreases, compared to the less good ones. This work has not yet been published, but we’ll look forward to the details.

Painted Butterflies–Rare Good News
Most of the news for butterflies is terrible, with monarchs and others in exponential decline. But for one species, the painted ladies swarm by the billions past LA.
www.latimes.com/science/sciencenow/la-sci-sn-butterflies-desert-explosion-20190312-story.html
Of course this species is a generalist, feeding on any sort of plant. And it’s common for environmental disruption to favor certain populations for overgrowth, while many more kinds decline, so diversity loses. Nevertheless, it’s breathtaking to see these little wings migrate up the coast.
To help the butterflies and the bees, I support the Xerces Society.
Sugar-Coated Nanomachines Cure Cancer
An idea going back to Fantastic Voyage is that physicians can shrink to microscopic size and travel through the blood vessels to treat the site of a patient’s illness. Today, we’re not shrinking human physicians, but nanomachines. This research team at University of Tokyo focuses on a particular challenge for nanomedicine, getting the therapeutic agents across the blood brain barrier. The blood brain barrier is especially challenging to fight brain tumors, such as glioblastoma, the kind that Senator McCain had. Dr. Kataoka discusses his work here.
Kataoka’s group used an ingenious trick to get their device across the blood vessel membrane. The capillaries into the brain exclude lots of things, but need nutrients—especially the sugar called glucose. The brain has one of the highest glucose uptake rates found in the body. The glucose is taken up by a protein called GLUT1 that is embedded in the membrane of the capillary cells. So the researchers built a delivery device called a “micelle” (basically a highly engineered soap bubble). The micelle contains sugar molecules attached to a carbon chaine (hydrocarbon) that dissolves into the micelle membrane. Now the whole sugar-coated object can bind to GLUT1 molecules in the capillary, and dissolve through the membrane.
How do we know it works? This micrograph shows the capillaries within the brain of a mouse. The sugar-coated micelles have a tagged molecule that fluoresces red. In the first image, we see the micelles only found within the capillary vessels. But after 60 minutes, the micelles have leaked out of the vessels into the surrounding tissue; a process known by a mouthfull of a term, “extravasation.” Extravasation is something that white blood cells normally do all the time, in most parts of the body, but not the brain.
If that’s not strange enough, at ICFA “Clone with Joan” Saturday breakfast we’ll hear more about how gut bacteria may take up residence in the brain (a controversial report) and how bacteria can treat human genetic diseases. Sounds more and more like the microbial aliens of Brain Plague.